Laboratory:Akiruno

Laboratory:Akiruno
CODE:00668 0
Please be sure to request this at the same time as culture identification.
☆ For sensitive drugs, we use our standard drugs.
Note) Depending on the details of the inspection requested, it may take more days.
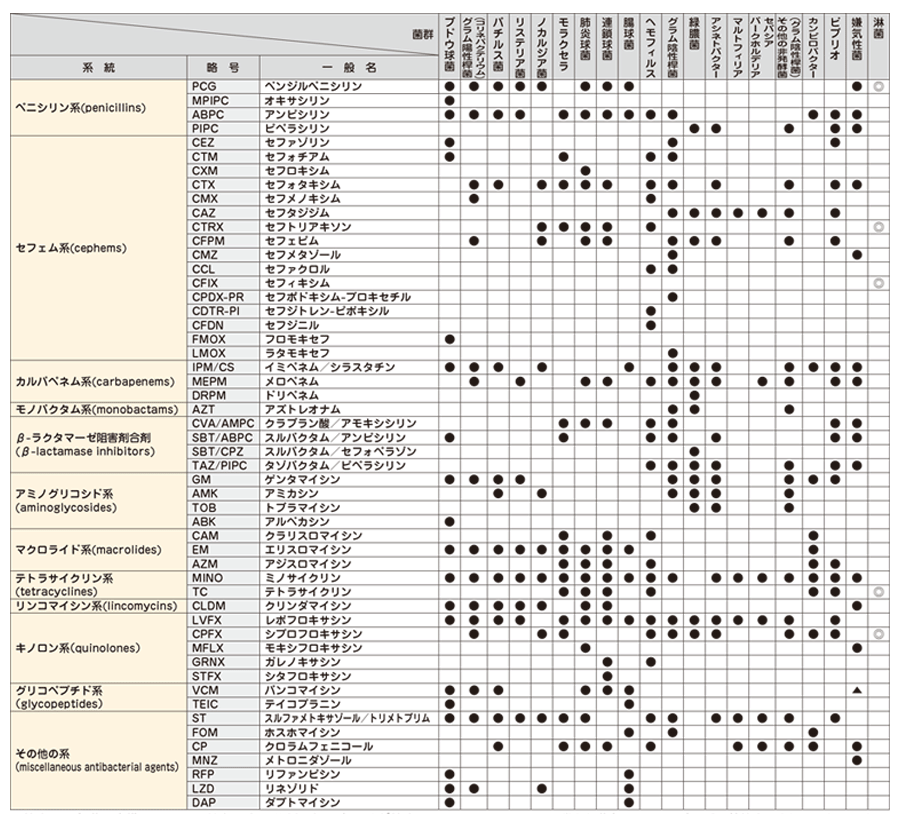
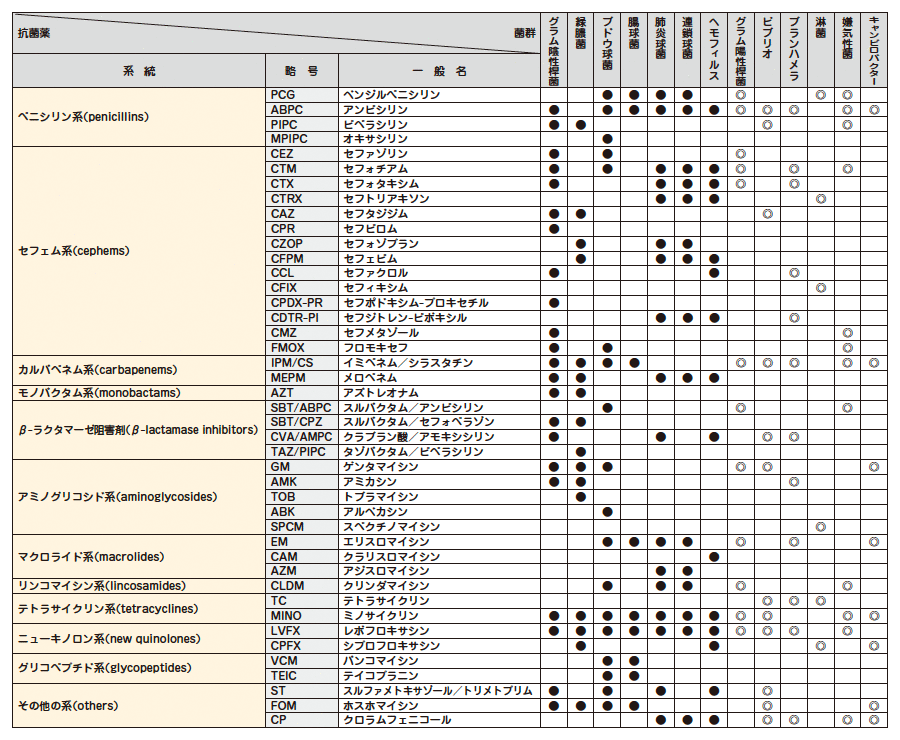
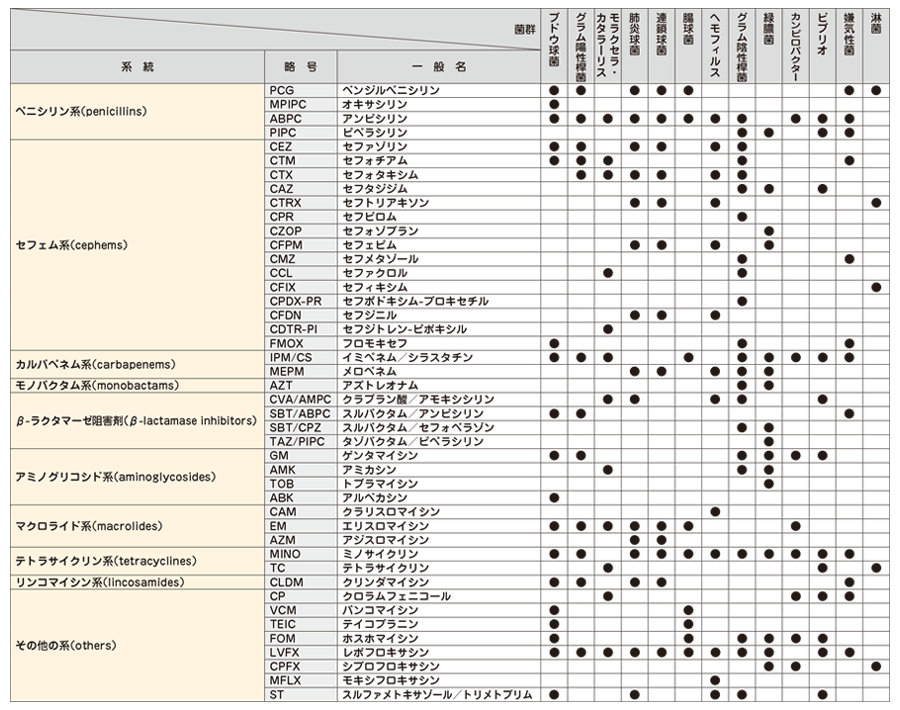
下記項目の検査結果は、次の当社基準に基づき、下記表示方法にて報告します。
(参考 : Clinical Microbiology Procedures Handbook - 4th edition, 2016.)
| 項目名 | 区分および鏡検倍率 | 表示方法 | 細菌数/細胞数 | 備考 |
|---|---|---|---|---|
| 塗抹鏡検 | 細菌数※ (鏡検倍率1,000倍) |
(-) | 菌がみられない | |
| 1+ | 1視野 <1 | |||
| 2+ | 1視野 1~5 | |||
| 3+ | 1視野 6~30 | |||
| 4+ | 1視野 >30 | |||
| 細胞数 (白血球・上皮細胞) (鏡検倍率100倍) |
(-) | 細胞がみられない | ||
| 1+ | 1視野 <1 | |||
| 2+ | 1視野 1~9 | |||
| 3+ | 1視野 10~25 | |||
| 4+ | 1視野 >25 |
※カルチャーボトルおよび菌株でご依頼した際、細菌数の表示方法は「陽性」となります。
| 項目名 | 表示方法 | 培地発育状態 | 備考 |
|---|---|---|---|
| 培養同定 嫌気性培養 |
(-) | 未発育 | 菌種名は、細菌命名に関する国際規約に基づき報告します。 |
| 1+ | 1/3未満 | ||
| 2+ | 1/3以上2/3未満 | ||
| 3+ | 2/3以上 | ||
| 4+ | 培地全面 |
| 項目名 | 区分および鏡検倍率 | 表示方法 | 細菌数 | 備考 |
|---|---|---|---|---|
| 塗抹鏡検 | 細菌数 (鏡検倍率1,000倍) |
(-) | 菌がみられない | |
| 1+ | 1視野 <1 | |||
| 2+ | 1視野 1~5 | |||
| 3+ | 1視野 6~30 | |||
| 4+ | 1視野 >30 |
| 項目名 | 表示方法 | 培地発育状態 | 備考 |
|---|---|---|---|
| 培養同定 嫌気性培養 |
(-) | 未発育 | 菌種名は、細菌命名に関する国際規約に基づき報告します。 |
| 1+ | 1/3未満 | ||
| 2+ | 1/3以上2/3未満 | ||
| 3+ | 2/3以上 | ||
| 4+ | 培地全面 |
| 項目名 | 区分および鏡検倍率 | 表示方法 | 細菌数/細胞数 | 備考 |
|---|---|---|---|---|
| 塗抹鏡検 | 細菌数 (鏡検倍率1,000倍) |
(-) | 菌がみられない | |
| 1+ | 1視野 <1 | |||
| 2+ | 1視野 1~5 | |||
| 3+ | 1視野 6~30 | |||
| 4+ | 1視野 >30 | |||
| 細胞数 (白血球・上皮細胞) (鏡検倍率100倍) |
(-) | 細胞がみられない | ||
| 1+ | 1視野 <1 | |||
| 2+ | 1視野 1~9 | |||
| 3+ | 1視野 10~25 | |||
| 4+ | 1視野 >25 |
| 項目名 | 表示方法 | 培地発育状態 | 備考 |
|---|---|---|---|
| 培養同定 | (-) | 未発育 | 菌種名は、細菌命名に関する国際規約に基づき報告します。 |
| 1+ | 1/3未満 | ||
| 2+ | 1/3以上2/3未満 | ||
| 3+ | 2/3以上 | ||
| 4+ | 培地全面 |
| 項目名 | 表示方法 | 内容 | 備考 |
|---|---|---|---|
| 薬剤感受性検査 | S | Susceptible (感性) | CLSIの基準に基づき表示します。 |
| I | Intermediate (中間) | ||
| R | Resistant (耐性) |
| 検査材料 | 検体量 | 容器 | 保存 | 採取方法 | |
|---|---|---|---|---|---|
| 抗酸菌 | 喀痰 | 2~3mL | X00 滅菌喀痰採取容器 | 上気道・口腔内常在菌により、できるかぎり汚染されないように滅菌喀痰採取容器に採取し、直ちに冷蔵保存してください。 (患者に充分説明し、うがいにより清潔にした後に、咳によって深部から喀出された、しかも唾液混入の少ない喀痰を採取してください。) |
|
| 便 | 小指頭大 | ARR 滅菌ポリスピッツ | 適量を無菌的に採取し、滅菌ポリスピッツに入れ冷蔵保存してください。 ・便の塗抹鏡検は実施しておりません。 |
||
| 胸水 腹水 胃液 |
各5~10mL | ARR 滅菌ポリスピッツ | 各サンプルを無菌的に採取し、滅菌ポリスピッツに入れ、直ちに冷蔵保存してください。 ・一般細菌を同時に依頼される場合はシードチューブⅡをご使用ください。 |
||
| 部分尿 | 5~10mL | ARR 滅菌ポリスピッツ | 無菌的に採取し、滅菌ポリスピッツに入れ、直ちに冷蔵保存してください。 | ||
| 抗酸菌核酸同定 | 喀痰 | 2.0mL | X00 滅菌喀痰採取容器 | 核酸同定検査ではコンタミネーションの影響が大きくなりますので検体採取にあたっては、取り扱いに充分ご注意ください。 上気道・口腔内常在菌により、できるかぎり汚染されないように採取し、冷蔵で提出してください。 |
ご依頼の際は、必ず材料・由来を明記してください。
| 分類 (由来) |
材料 | 検体量 | 容 器 | 保存 | ||
|---|---|---|---|---|---|---|
| 一般細菌 | 口腔 ・ 気道 ・ 呼吸器 |
喀痰 | 2~3mL | X00 滅菌喀痰採取容器 | 上気道・口腔内常在菌により、できるかぎり汚染されないように滅菌喀痰採取容器に採取し、直ちに冷蔵保存してください。 (患者に充分説明し、うがいにより清潔にした後に、咳によって深部から喀出された、しかも唾液混入の少ない喀痰を採取してください。) | |
| 咽頭粘液 | 適量 | VS1 eSwab105 レギュラーFLOQスワブ | eSwab105 レギュラーFLOQスワブの綿棒で咽頭をこすって、粘液を採取し、直ちに付属のアミーズ培地に無菌的に入れ、速やかに冷蔵保存してください。 | |||
| 消化管 | 便 | 適量 | VS1 eSwab105 レギュラーFLOQスワブ | eSwab105 レギュラーFLOQスワブを用い適量の便を採取し付属のアミーズ培地に無菌的に入れてください。 ・クロストリジオイデス・ディフィシルの検査にはシードチューブⅡを使用してください。 ・便の塗抹鏡検は実施しておりません。 |
||
| 胆汁 (A・B・C) 胃液 十二指腸液 |
各5~10mL | C10 シードチューブⅡ | 無菌的に採取し、シードチューブⅡに速やかに注入し、直ちに冷蔵保存してください。 | |||
| 泌尿器 ・ 生殖器 |
部分尿 尿管カテーテル 膀胱カテーテル |
各5~10mL | ARR 滅菌ポリスピッツ | ・無菌的に採取し、滅菌ポリスピッツに入れ、直ちに冷蔵保存してください。 ・淋菌の検索を目的とする場合は室温保存してください。 ・トリコモナスの検索を目的とする場合は室温保存してください。 |
||
| 前立腺液 腟分泌物 膿 |
各適量 | VS1 eSwab105 レギュラーFLOQスワブ | eSwab105 レギュラーFLOQスワブの綿棒で適量を採取し、付属のアミーズ培地に入れ、直ちに冷蔵保存してください。 ・淋菌の検索を目的とする場合は室温保存してください。 ・トリコモナスの検索を目的とする場合は室温保存してください。 |
|||
| 血液 ・ 穿刺液 |
動脈血 静脈血 |
各3~10mL (最適量8~10mL) |
CBG、CBS カルチャーボトル |
カルチャーボトル好気性菌用CBS、嫌気性菌用CBGの2本にそれぞれ3~10mLの血液を無菌的に注入し、緩やかに転倒混和し、室温保存してください。 フラン器での保存は検査結果に影響を与えます。 | ||
| 髄液 腰椎穿刺液 |
各3~5mL | C10 シードチューブⅡ | 3~5mLのサンプルを無菌的に採取し、シードチューブⅡに速やかに注入し、室温保存してください。 | |||
| 胸水 腹水 関節液 |
各5~10mL | C10 シードチューブⅡ | 5~10mLの各サンプルを無菌的に採取し、シードチューブⅡに速やかに注入し、直ちに冷蔵保存してください。 | |||
| その他 | 耳漏 | 適量 | VS1 eSwab105 レギュラーFLOQスワブ | eSwab105 レギュラーFLOQスワブの綿棒で適量を無菌的に採取し、付属のアミーズ培地に入れ、直ちに冷蔵保存してください。 | ||
| 皮膚 爪 毛髪 |
各適量 | ARR 滅菌ポリスピッツ | 各適量を無菌的に採取し、滅菌ポリスピッツに入れ、直ちに冷蔵保存してください。 ・真菌検査のみ実施いたします。 |
|||
| 組織 膿 |
各適量 | C10 シードチューブⅡ | 各サンプルを綿棒で無菌的に採取し、シードチューブⅡに速やかに入れ、直ちに冷蔵保存してください。 | |||
| 他 | 便 (CDトキシン(GDH)) | 1.0g | F00 糞便容器 | 単独の検体にてご依頼ください。 |
| 分類 | 材料 | 検体量 (mL) | 保存条件 | 採取容器 | |
|---|---|---|---|---|---|
| 口腔・気道・呼吸器 | 喀痰 | 2 ~ 3 5 ~ 7 | X00 | 滅菌喀痰採取容器 | |
| 咽頭ぬぐい液 | 適量 | VS1 | eSwab105 レギュラーFLOQスワブ | ||
| 消化管 | 便 | 適量 | F01 VS1 | キャリブレアー採便管 eSwab105 レギュラーFLOQスワブ | |
| 胆汁 | 5 ~ 10 | ARR XR | 滅菌スピッツ10mL用 | ||
| 胃液 | 2 ~ 3 5 ~ 10 | ARR XR | 滅菌スピッツ10mL用 | ||
| 泌尿器・生殖器 | 中間尿 | 5 ~ 10 | ARR XR | 滅菌スピッツ10mL用 | |
| カテーテル尿 | 5 ~ 10 | ARR XR | 滅菌スピッツ10mL用 | ||
| 腟分泌物 | 適量 | VS1 | eSwab105 レギュラーFLOQスワブ | ||
| 膿 | 適量 | VS1 | eSwab105 レギュラーFLOQスワブ | ||
| 血液・穿刺液 | 静脈血 | 最適量 8 ~ 10 |
CBG CBS |
血液カルチャーボトル | |
| 動脈血 | CBG CBS |
血液カルチャーボトル | |||
| 髄液 | 2 ~ 3 3 ~ 5 | C10 | シードチューブⅡ | ||
| 胸水 | 5 ~ 10 | C10 | シードチューブⅡ | ||
| 腹水 | 5 ~ 10 | C10 | シードチューブⅡ | ||
| 関節液 | 5 ~ 10 | C10 | シードチューブⅡ | ||
| その他の部位 | 耳漏 | 適量 | VS2 | eSwab106 ミニチップFLOQスワブ | |
| 眼脂 | 適量 | VS1 | eSwab105 レギュラーFLOQスワブ | ||
| 皮膚 | 適量 | ARR XR | 滅菌スピッツ10mL用 | ||
| 爪 | 適量 | ARR XR | 滅菌スピッツ10mL用 | ||
| 組織 | 適量 | ARR XR | 滅菌スピッツ10mL用 | ||
| 膿 | 適量 | C10 | シードチューブⅡ | ||
| 他 | 環境材料 | - | C30 | スタンプ培地 (院内環境検査用) | |
| - | VS1 | eSwab105 レギュラーFLOQスワブ | |||
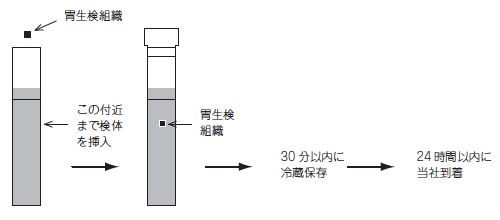
| 胃生検組織 | 適量 | C20 | シードチューブHP (ヘリコバクター培養同定用) | ||
| 便 | 1.0g | F00 | 糞便容器 | ||
| 他 | 胃生検組織 | 適量 | C20 | シードチューブHP (ヘリコバクター培養同定用) | |
| 便 | 1.0g | F00 | 糞便容器 | ||

滅菌ポリスピッツ 10mL用
貯蔵方法:室温

血液培養ボトル (嫌気用)
抗生物質吸着中和剤入
貯蔵方法:室温
有効期間:製造から9ヵ月
最適量:8~10mL (許容範囲 3~10mL)

血液培養ボトル (好気用)
抗生物質吸着中和剤入
貯蔵方法:室温
有効期間:製造から9ヵ月
最適量:8~10mL (許容範囲 3~10mL)

血液培養ボトル (小児用)
抗生物質吸着中和剤入
貯蔵方法:室温
有効期間:製造から9ヵ月
採取量:1~3mL

シードチューブⅡ 嫌気培養用 (容器容量4mL)
内容:寒天、塩化ナトリウム、リン酸塩等
貯蔵方法:室温
有効期間:製造から6ヵ月

シードチューブHP (ヘリコバクター培養同定用)
貯蔵方法:冷蔵
有効期間:製造から3ヵ月

スタンプ培地 院内環境検査用
貯蔵方法:冷蔵
有効期間:製造から4ヵ月

糞便容器
貯蔵方法:室温

キャリーブレア採便管
貯蔵方法:室温
有効期間:製造から6ヵ月

eSwab105 レギュラーFLOQスワブ
内容:アミーズ培地 1mL
貯蔵方法:室温
有効期間:製造から1年3ヵ月

eSwab106 ミニチップFLOQスワブ
内容:アミーズ培地 1mL
貯蔵方法:室温
有効期間:製造から1年3ヵ月

滅菌喀痰採取容器
貯蔵方法:室温

滅菌喀痰採取容器
貯蔵方法:室温

滅菌ポリスピッツ
貯蔵方法:室温


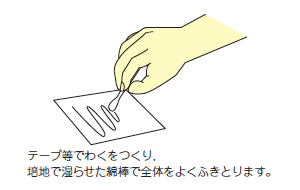
被検体の一定面積 (10cm×10cm通例) を eSwab105 レギュラーFLOQスワブに添付されている綿棒 (あらかじめ培地に刺して湿らせておく) にてよくふきとり 再度培地に刺しご提出ください。
スタンプ培地 (C30) のキャップをとりはずし、 培地面に手指など触れないよう注意し 被検体の表面に培地面をかるく押しつけます。 約5秒後再びキャップをしてご提出ください。