Laboratory:Akiruno

Laboratory:Akiruno
CODE:06471 2
Real-time PCR
A type of nucleic acid amplification method based on the basic principle of PCR, which uses oligonucleotides that emit fluorescence upon decomposition to quantify target nucleic acids in real time by checking the fluorescent signal at each PCR cycle. A measurement method that enables
![]()
The measurement targets are 16 type, 18 type, and other high risk groups (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 type, and 66 type). Regarding ""Other High Risk Groups"", it is not determined by type. Please note that if the sample contains blood, it may affect the data. With this testing method, the affects of contamination are greater, so please be careful when handling the specimen.
[06471 2] Human papillomavirus DNA (type 16, type 18, and other high risk groups), [06487 5] About human papillomavirus (HPV) genotype determination
[02326 7] Cytodiagnosis (gynecology LBC) Or [06241 7]Cytodiagnosis (Gynecology LBC Bethesda System) can only be requested at the same time, but in that case, the required time for cytodiagnosis will be delayed by 1-2 days.
内容:メタノール55%
貯蔵方法:室温
有効期間:製造から1年6ヵ月
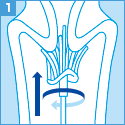
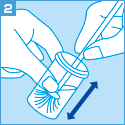

[ご注意]
妊婦より細胞を採取する場合は、安全性を考慮し、ブラシ、スパーテル等などの採取器具の使用は避け、綿棒を使用してください。
ただし、綿棒で検査に必要な細胞量を採取するために、採取前に別の綿棒で粘液を除去し、
採取に使用した綿棒を保存液中で充分にすすぎ、採取した細胞を洗い落としてください。
容器には綿棒の先端を残さないで室温保存してください。
また、綿棒では無理な力がかかりますと折れる可能性がありますので充分にご注意ください。