Laboratory:Akiruno

Laboratory:Akiruno
CODE:00W58 5
Real-time PCR
A type of nucleic acid amplification method based on the basic principle of PCR, which uses oligonucleotides that emit fluorescence upon decomposition to quantify target nucleic acids in real time by checking the fluorescent signal at each PCR cycle. A measurement method that enables
![]()
Requests cannot be made for containers other than the designated container (V41).
Please note that if the sample is contaminated with blood, the data may be affected.
This testing method is more susceptible to contamination, so please be careful when handling samples.
*Notes
-This is a test item for cervical cancer screening using HPV testing alone.
-If the result of this test is ”positive (+)”, an additional ”gynecological cytology test (triage)” will be performed.
-The required number of days for ”gynecological cytology (triage)” is 4 to 7 days.
-We use Roche Diagnostics' ”Cobas ®6800/8800 System HPV.”
The targets for measurement are types 16,18,and other high-risk groups (types 31,33,35,39,45,51,52,56,58,59,68,and 66).
-Samples will not be returned.
内容:メタノール55%
貯蔵方法:室温
有効期間:製造から1年6ヵ月
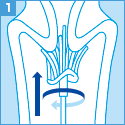
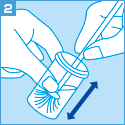

[ご注意]
妊婦より細胞を採取する場合、使用できる採取器具等に制限があります。
サーベックスブラシは妊娠10週以降禁忌、
サイトピックの子宮頸管内検体採取用端子は妊娠週数にかかわらず禁忌となりますのでご留意ください。
なお、綿棒は使用しないでください。

※ HPV核酸検出 (検診) のみをご希望の場合、担当営業員へご相談ください。
HPV陽性検体のみの検体返却が可能です。